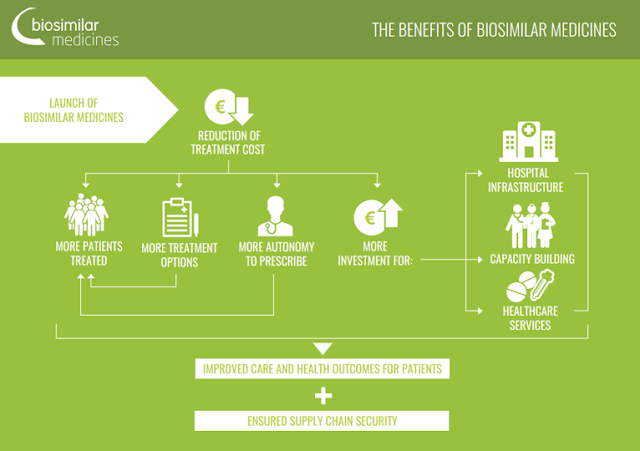
Well Being and Fitness Tips for Men and Women
Top Well being Tips for Males and Females
In day today's life every one is busy with their stuffs and they usually don't get time to look after their health and to take care of themselves. Here are some simple fitness tips for mens and womens to better their health and well being:
1. Eat solid edibles
Nutritious food varieties give you energy and may lessen your
possibilities getting specific infections. Incorporate vegetables, foods grown
from the ground free or low-fat milk items.
2. Maintain a solid body mass
Being large or overweight can expand your possibilities getting a stroke, coronary illness, hypertension and diabetes.
Discover your weight file (BMI-Body Mass Index) to check whether or not you are in danger.
Along these lines,
get more dynamic, eat good food varieties and control your piece sizes.
3. Get moving
Normal actual work is very fundamental for your well being.
Look for a decent simple exercise to begin with.
4. Be sans smoke
Smoking is related with a significant number of the main
sources of death, like stroke, lung illness and disease. Additionally, swear
off recycled smoke.
5. Get standard screening and tests
Get some information about how frequently you expect to be
inspected. Continuously request evaluating tests for certain circumstances and
sicknesses, including physically sent diseases, diabetes, elevated cholesterol,
specific kinds of malignant growth and hypertension.
6. Manage pressure
Adjusting family and work commitments can be extreme.
Nonetheless, it is fundamental to safeguard your physical and mental prosperity.
Track down solid choices to assist you with adapting to pressure.
7. Getting sufficient rest
Absence of appropriate rest can influence your state of mind
and your well being. Check with your wellbeing specialist in the event that you
think you have a major issue.
8. Know your dangers
Figure out what your way of life might mean for your gamble of medical conditions. For example, men who work with specific synthetic substances expect to make defensive strides.
Figure out how to monitor your
family clinical history and talk about this with your well being professional.
9. Stay safe
Security represents numerous things, for example, wearing head protectors, safety belts, keeping well being guidelines at work and furthermore having working smoke alarms.
It likewise implies utilizing
insurance during sex, wearing sunscreen, cleaning up routinely and dealing with
your teeth.
It's vital to practice routinely. While that is been sound guidance for a long time, unfortunately exceptionally a few people are following it.
Taking into account that in the wake of missing fourteen days of activity
schedules limit begins to diminish, obviously practice is the way to progress.
Here you will observe extreme aide for health and
wellbeing tips and you will figure out how to carry on with more
solid and fit way of life.
Numerous people are normally really meager and can likewise appears to be a piece off-kilter.
You can put it on hereditary variables or
whatever, but their body simply won't add any mass.
Wellness has never been of much significance in the present
life where both the people are enjoying the multiple ways of hurting his/her
body; smoking, drinking, unhealthy food with no actual work or exercise, dozing
late around evening time and moreover. However, human is adequately shrewd to
track down the ways of including all out wellness into their lives to conquer
impacts of the pressure.
●
Peruse Product Labels
Basic, correct? Anyway we all don't get it done. We base our choices on a case shows up and factors like expense. The thing mark records the quantity of the sustenance and calories, the food contains.
It's genuinely easy
to acknowledge how much sugar, fat, and fake fixings each thing involves. Being
educated is the underlying advance toward a far solid eating regimen.
If you want to put on weight, simply consume 500 extra
calories every day, except remember to initially design a healthfully balance
diet containing proper measures of proteins, starches and fats along with
miniature supplements like minerals and nutrients.
Also, simply keep away from healthfully poor/void food. You could wind up putting weight at some unacceptable spots, as well as raising your cholesterol and disturbing your blood insulin levels.
Monitoring the number of
calories you eat in a day will be useful in the preparation out your actual
working out.
At any point can't help thinking about why the muscle heads' weights are so large? That is on the grounds that they plan out their suppers and take in more solid calories than a normal individual.
Then again, shedding
pounds and making progress toward a skinnier build will include more actual
exercise than the calories you ingest.
●
Keep away from Canned or
Frozen Food
I realize these merchandise simplify life. In any case,
buying your food new suggests it - lower in compounds, more extravagant in
supplements, and by and large better.
●
Make certain to Get
Enough Sleep
Despite the fact that the vast majority of us have eight-hour
occupations during day or night, it's essential to get sufficient rest to
re-energize our body's batteries. Six to eight hours of rest will move your
body along over the course of the day.
Yet, assuming you end up feeling tired anytime subsequent to returning home from the work, by all means lay down for a little rest before the working out.
You should just rest for about thirty minutes. This will keep
you from remaining up later in night.
●
Drink Plenty of Water
What you drink is as vital to your wellbeing. However, you want to guarantee that you're not drinking extra-calories.
A common rule is to
drink refreshments, that are plain. Creator espressos, refreshments, and sugar
drinks contain sugar substitution, a lot of sugar, or calories.
Drinking sufficient water is one of most significant wellness tips. It is suggested that one should drink somewhere around 3-4 liters of water each day.
Water keeps up with the level of our organic liquids, scrubs
our framework and hydrates our skin.
●
Practice Daily
You should exercise day by day for at minimum 60 minutes. You don't need to off yourself from running, running, and so forth, however you ought to have some kind of moderate actual work or action in your day to day existence and schedule.
Assuming you are hoping to shed a couple of pounds
quick, do a more elevated level power exercise or exercise.
For instance, go on a stroll at a lively speed for no less than 60 minutes. Or on the other hand, you can run and set the specific stretches to run during that hour.
Ensure you're not in the extreme torment
during your exercise.
It very well might be bothering, yet it implies that your
body is improving. Make certain to remain hydrated, stretch, and eat food
varieties with a nice measure of the protein after every exercise.
●
Nibble Around
Have 3 snacks per day in the middle of the 3 dinners.
A mix
of 3 dinners and 3 snacks a day is perfect. Simply make sure to remain off
undesirable tidbits.
Instead, you can attempt nutritious food sources like nuts,
seeds, natural products, cheddar, and so forth.
●
Wellness Diet
Food choices
The thought is to pack in calories and put on weight without heaping on overabundance weight.
Pepper your eating routine with healthfully
rich food sources like potatoes, corn, earthy colored rice, earthy colored
bread, bananas alongside other such fatty food sources.
a). Keep up with the wellness diet with vegetables, organic
products, proteins, starches, unsaturated fats, mono-unsaturated fat,
poly-unsaturated fat and different supplements.
b). Keep away from handled food, unhealthy food and other
food with the soaked and trans-fat substance.
Your dinner should incorporate a wide scope of food varieties
- bland food varieties, protein rich food varieties, calorie rich food sources,
stimulating oils, and a few food sources wealthy in fiber, similar to vegetables
and natural products.
●
Eat More Often
Taking 3 gigantic suppers and more prominent spans, it is
experimentally demonstrated that 5 more modest dinners at 4 hour stretches
would be better and is the secret to get you far from the gorging.
It is additionally extremely indispensable to eat lesser in
these suppers as day advances.
●
Follow A Healthy
Lifestyle
Try not to smoke, drinking, handled food, low quality food,
food varieties with elevated cholesterol, salt, sugar and the oil.
●
Try not to Skip Your
Meals
You ought to never avoid your suppers which is actually an
unfortunate quirk. Try not to avoid 3 day by day suggested suppers - breakfast,
lunch and supper.
Make your morning meal and lunch huge, yet keep the supper
somewhat light, as dozing on a full midsection isn't really great for well being
by and large. It ruins your metabolic cycle and makes it more challenging to
process to additional dinners.
In the event that you regularly avoid your morning meal, you
might be set out toward inconvenience, says Leah Cahill, PhD, of the Harvard
School of Public Health.
"As we rest all
the night we are fasting, thus in the event that we consistently don't 'break
quick' toward the beginning of the day, it places a strain on our bodies that
throughout the time can prompt the sort 2 diabetes, insulin obstruction and
pulse issues."
Assuming you are thinking about fasting consistently, talk
with an enlisted dietitian or other sustenance genius to guarantee that you're
getting an adequate number of nutrients, protein, minerals, and fundamental
unsaturated fats in your bites and dinners.
●
Try not to Wait Till You
Are Hungry
One of the most essential signals your body conveys is the
one for hunger. That natural stomach snarl tells us it is an ideal opportunity
to eat something. Ghrelin, the body's craving chemical, is delivered in
pancreas and the stomach coating and attempts to animate hunger.
Regardless of whether you incline toward 6 more modest dinners or 3 primary suppers daily, listen constantly to your body letting you know it's full. In the event that you have furnished it with the enough energy, it will tell you, normally inside 20 minutes after your supper time.
Eat before
you feel food cravings, since you will quite often eat more than the necessary
sum when you feel hunger.
Whenever our glucose drops genuinely low, we will generally
get all the food we can get our hands on. This is clearly not great when you
are attempting to lose your weight.
Wellbeing and Fitness Tips For Women
Despite the fact that there are numerous logical reasons that
men are more grounded than the ladies, it doesn't imply that wellness practices
and the exercise are just implied for men.
● The following are the
wellness tips for the ladies that set them up well towards the actual
activities and exercises.
● The bones of ladies are inclined to become more vulnerable and more delicate when they step in their 30s.
It's essential that they take unique consideration of their bones, and
attempt to remember more calcium for their eating routine.
● Focusing on your eating
regimen, and never avoid your suppers in anticipation of shedding pounds
faster. IT NEVER WORKS AT ALL.
● Incorporate iron and the
folic acids into your eating regimen for better blood flow, by polishing off a
greater amount of green verdant vegetables, juices, beans, chicken, and so on
● Snare on to the cardio,
either by joining a rec center or basically running or climbing the steps. Any
sort of cardio is really great for you.
● Get a bit of 'personal
time', to renew your energy and re-energize your psyche and the body. Go out
for a walk, Read a book, or pay attention to alleviating music. Anything causes
you to feel loose.
● Eliminate the liquor, as
it has never done well to both of genders. Ladies are prescribed not to polish
off in excess of 14 units of the liquor seven days (contingent upon the
strength of alcohol).
Final Words
The article mentions how the boys and girls will take care of their health and wellness.
Hope you will like the
tips and if you like it please feel free to comment...